How Strategic Scientific Communication Sets Companies Apart
In the fast-paced world of biotech innovation, your science doesn’t speak for itself — how you communicate it does. From securing funding to regulatory approvals, strategic scientific communication is a growth lever that early-stage and clinical-stage biotech companies must plan for deliberately. At BioVertex Consulting, we believe that with the right planning, your data becomes a story that drives momentum.
Tanushree Phadke
6/27/20251 min read
Why Biotech Startups Need a Scientific Communications Strategy
1. Timing Is Everything- Communications should be synchronized with key development milestones:
IND filing
Trial initiation or readouts
Strategic partnership announcements
IP or publication disclosures
Mistimed messaging (e.g., releasing clinical data before patent filing) can be a reputational or commercial risk.
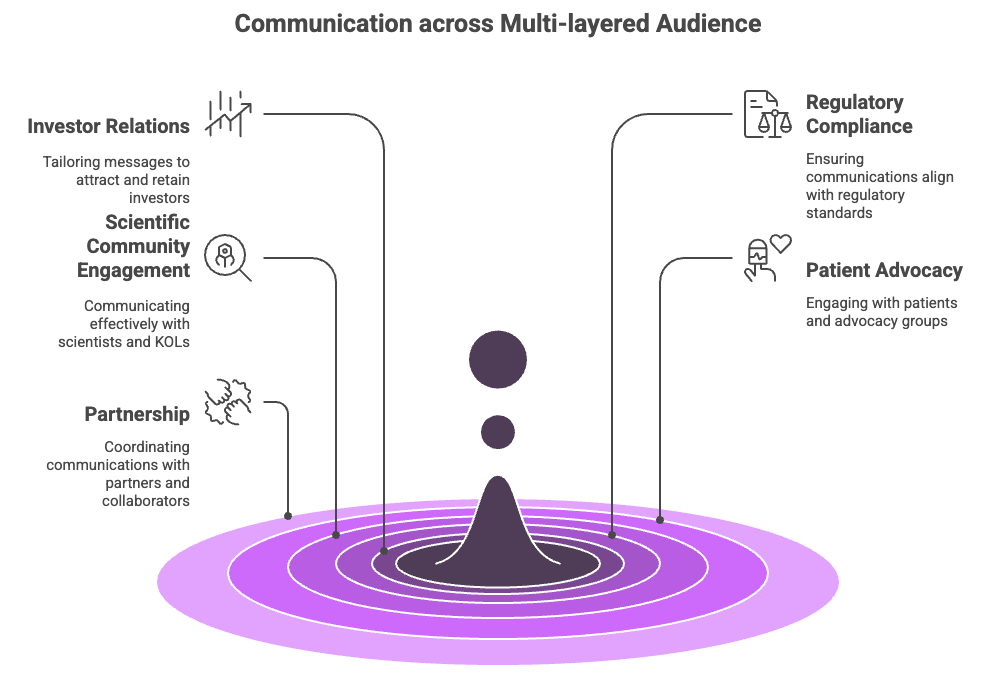
2. Your Audience Is Multi-Layered- The same data means different things to:
Investors
Regulatory reviewers
Scientists & KOLs
Patients & advocacy groups
Partners & collaborators
Without a plan, messages become inconsistent. And inconsistency erodes credibility and trust.
What Should a Strategic Communications Plan Include?
✅ Messaging Framework
Use these prompts to shape your biotech story:
What problem are we solving?
What’s the scientific innovation?
What’s the clinical or business impact?
Why now?
Tip: Boil this down into a 3-slide narrative every leader on your team can tell the same way.
✅ Multi-Channel Content Strategy
Reach your audiences via:
Scientific conferences and abstracts
Peer-reviewed publications
LinkedIn and company blog content
Regulatory and investor presentations
KOL advisory boards
Patient-focused webinars
Earned media or trade press
Tip: Your communication plan should define who needs to hear what, when, and through which channels.
✅ Governance & Workflow
Establish framework to determine:
Who signs off on scientific claims, visuals, and public statements?
How do you ensure version control, especially with live documents?
Do you have cross-functional input (R&D, Regulatory, Legal, Comms) at the right time?
Tip: Create a communications calendar that mirrors your development roadmap.
Final Thought: In Biotech, Communication Is Strategy. Your data is powerful, but only when translated effectively.
📣 To learn how BioVertex Consulting can support your communication strategy planning reach out-
📩 contactus@biovertexconsulting.com
🌐 https://www.biovertexconsulting.com
Let's navigate the complexities of life sciences, together.
Contact Us
© 2025. All rights reserved.
